I have a pet-peeve of seeing perfectly good medicines being thrown in the trash because of non-medical concerns. Usually, the FDA is the bureaucratic puppet whose final job it is to justify and instill the “This Drug Is Dangerous” label. Hydroxychloroquine is one of those drugs. It is important to lead you through the story of how one drug can go from development, long-term use – so long that it becomes generic but so useful, safe, and profitable, companies will still manufacture it – to a place where a physician prescribing this could severely hurt someone.
Hydroxychloroquine is not a cure
If one follows many blogs and article critiquing about the use of hydroxychloroquine, a main critique is that “it is not a cure”. The writers are correct. There is not virus killing drug. Not drug binds to “THE” virus and makes is shrivel up or implode. We all must be careful not to use this language. The hope and purpose of the Hydroxychloroquine is to slow or halt the replication of the virus in the body.
“SCIENCE” writers writing about science.
I came across an article that I will use to outline my deliberation. It has a nice review of the timeline of Hydroxychloroquine as a drug and the fiasco of using this as a possible treatment for Coronavirus ( COVID-19). Of all places, this was written in WIRED. The Science writer is not self-aware of his own bias, but a good outline still. Using the review written by Adam Rogers, a “science and geeky stuff” writer; ( He has no background in medicine, virology, epidemiology, statistics, infections diseases, or research as given in byline. Seems I better state that since that is a standard critique of anyone discussing Covid)

Then 2020…
Antivirals
Hydroxychloroquine is not a random apple picked off the tree of knowledge. It has a purpose in the possible treatment of persons with a virus. This does not mean a magic bullet cure of the virus. But that is not what anyone is looking for when it comes to viruses. Tamiflu (oseltamivir) is an oral “antiviral” medication routinely given to patients with early viral flu symptoms. It binds to the influenza virus once it is in a cell not allowing it to escape and invade other cells, allowing our own immune system to catch up. It does not “kill” the virus. This does not work on a coronavirus, therefore scientists in late 2019 into 2020 were looking for something to reduce the symptoms.
MEDCRAM
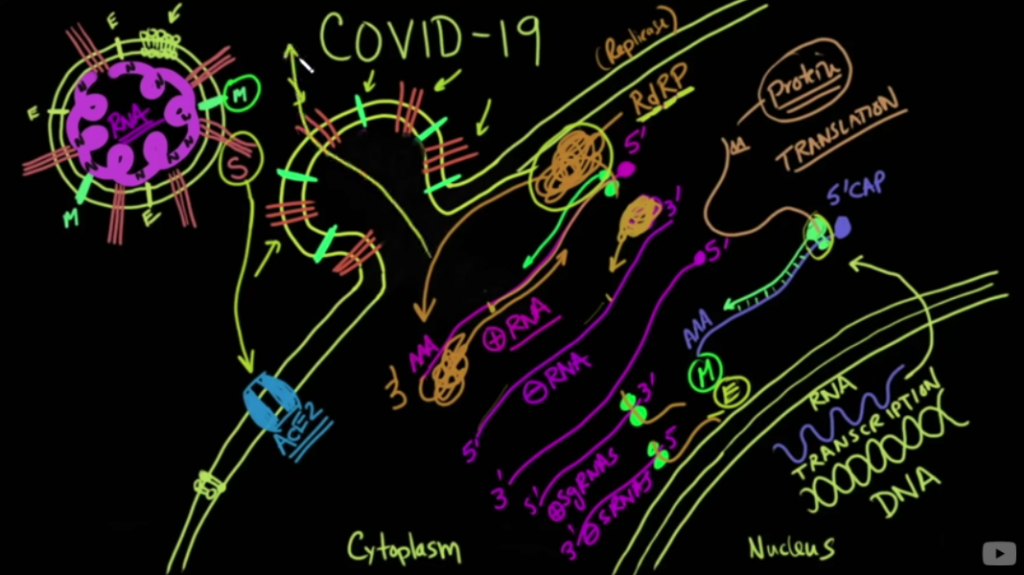
Medcram is an Internal Medicine CME review site that does wonderful free videos pertaining to a variety of medical subjects. Roger Seheult, MD began following the progress of the coronavirus spread and pandemic with daily to weekly videos. On March 6, 2020, he reviewed the molecular science of the coronavirus attacking a cell. This is important time-wise. ( I recommend these videos for extra knowledge,… otherwise, for the purpose of this article, you have to trust me, but I give you the evidence)
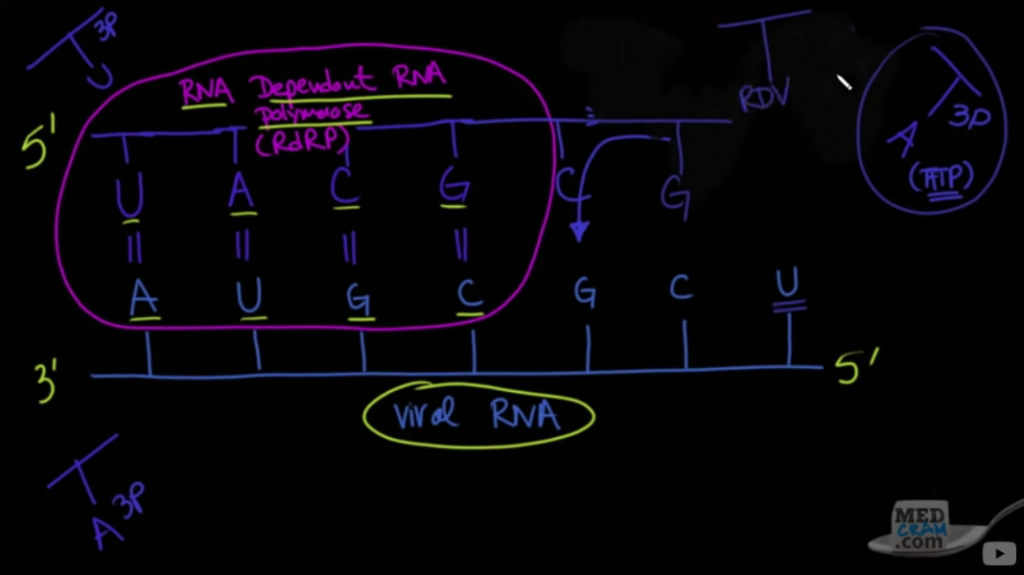
One key is the RNA polymerase which replicates the RNA of the virus in the human cell, many times the lung cells. In 2010, a study was done showing that Zinc can inhibit the coronavirus polymerase. SARS-CoV from 2002 was used in this study. This was only In-Vivo ( in the lab, not human) but clearly had a reason to be discussed. This virus also binds to the same ACE2 receptor which Covid-19 does. From the study;
Positive-stranded RNA (+RNA) viruses include many important pathogens. They have evolved a variety of replication strategies, but are unified in the fact that an RNA-dependent RNA polymerase (RdRp) functions as the core enzyme of their RNA-synthesizing machinery.
This essentially means, the virus RNA does not work without a working RNA polymerase. So at this time, since a YouTube page Foran internal Medicine review company understood the molecular biology of the Covid-19 ( coronavirus), Dr. Fauci and the team in the White House did also and were discussing it.
On March 9, 2020, Remdesivir was reviewed on Medcram as a possible RNA polymerase inhibitor and the biology behind this drug. This drug had made it through phase 1 and 2 trials, so relatively safe, but needed phase 3. But this medication would be much more expensive for patients and health care trying to do the same job as Hydroxychloroquine.
On March 10, 2020, Roger Seheult, MD reviewed some of the early science of Hydroxychloroquine. On March 18, 2020, a video mentioned the study by Professor Didier Raoult in France of 24 patients treated with Hydroxychloroquine. On March 19, 2020, MedCram reviewed the Coronavirus Taskforce Q&A with President Trump present, in which he discussed Hydroxychloroquine. The article in Wired refers to this in a way criticizing President Trump for being overly supportive of the drug. During the same Medcram video, it is discussed the University of Minnesota hydroxychloroquine study by Dr. David Boulware. The FAD and University research committee did not at all stand in the way of this proceeding.
By this time in March, many were discussing Chloroquine/ Hydroxychloroquine as a possible treatment. Biologically, this was not to kill the virus, not a cure, but to slow the spread in the body. President Trump could not be blamed for the hype of this medication. There was a biological reason to look at this drug.
James Todaro MD
In mid-March 2020, Dr. Todaro, along with cohorts, wrote a “google doc” review of the possible use of hydroxychloroquine for the treatment of COVID-19. This hit the internet by storm. But soon was shut down by Google and others. You can read the original doc and more at his website, Medicine Uncensored. This was not a medical research article – just a review of all that was going on with the use and research of Hydroxychloroquine for the treatment of COVID-19. Google erased his document, but this “paper” started the talk of possible help for persons who get infected.
Dr. Todaro graduated medical school from Columbia University and completed his surgical training residency in ophthalmology. Not too shabby. He now is an investor in Bitcoin. Before continuing I have to stop here and editorialize. Data makes evidence-based medicine. Dr. Todaro’s data may be incomplete and even show inadequate outcomes – but I do not think we ever reached an endpoint with Hydroxychloroquine. From WIRED;
The original Google Doc made a good case for chloroquine being of interest—attempted use in prior pandemics, studies in cells and in animals, preliminary results from China. Not proof, to be sure, but tantalizing hints.
I wonder if someday we may compare Dr. Todaro with another physician turned investor, Michael Burry, of the “The Big Short” fame, who saw the housing bubble no-one else did, shorted the market and won because he looked. In an April 6 article in Bloomberg, Dr. Burry discussed the potential costs of any lockdown and stated,
some controversial treatments for Covid-19, such as the malaria drug hydroxychloroquine, should be made more widely available.
If you understand finance, investing and Bitcoin, you can understand numbers, research, and epidemiology. People may make bad decisions, but Dr. Todaro will not make them because he is not intelligent.
March, 2020
Early March there was said to be a shortage of chloroquine and hydroxychloroquine. The United States actually had an emergency stockpile of this drug in case. It was a standard medical practice to use this in some New York hospitals. By mid-March, the US Food and Drug Administration consented to a prospective randomized trial for Hydroxychloroquine to be done at the University of Minnesota by David Boulware, professor of medicine and infectious disease scientist. From December 2019 to March 2020, Hydroxychloroquine was not gaining a reputation for side effects or causing deaths. At this time, the art of medicine and hope was in full throttle.
The Wired article goes into several aspects of behind the curtain government bureaucracy. Trump was the President and so the article points to him as as one of the antagonists for the hydroxychloroquine (HCQ) controversy. Though many in Big tech and other places, both left and right , were hyping the possibilities of HCQ.
Stephen Hahn, the Commissioner of the Food and Drug Administration (FDA)
This Wired article makes Mr. Hahn appear as cautious towards Hydroxychloroquine, which I can’t critic. But it does not mention this statement on the Today Show;
A doctor and a patient need to assess the data that’s out there, FDA does not regulate the practice of medicine, and that in the privacy of the doctor-patient relationship is where that decision should be made.
And what was not quoted from this Today Show interview was this statement; ( my transcription)
As you know these drugs (chloroquine and hydroxychloroquine) have been approved for a number of years for other indications by the FDA. We know they’re safe in those settings. but we want the right information out there to providers so they can make the right decision.
David Boulware MD
Boulware’s team proposed two large, double-blinded, randomized controlled trials. Per WIRED;
One would look at whether hydroxychloroquine could prevent illness in people with exposure to an infected person—“post-exposure prophylaxis”—and another would see if taking the drug close to the onset of symptoms could keep those symptoms from getting worse. That was “early treatment.”
The FDA approved the use of Hydroxychloroquine in Dr. Boulware’s trial at about the time Dr. Todaro’s Google doc paper came out. From there a storm of hope, enthusiasm, and early calls of a cure created a behind the scenes political fight. I could write a book on the possible industrial complex war on Hydrochlorquine, but the question I need to answer for you is if the possible authorities we trusted to ack truthfully did so.
Dr. Boulware was able to put together his trial and with difficulty from the WHO advising against anymore trials of Hydroxychloroquine, his trial had difficulty getting the number of patients to register for the trial. President Trump’s enthusiasm may not have helped the situation, as many on the LEFT did not want to see him succeed. It reminds me of George W Bush and 9/11. After that, he had the the next election won by looking successful in finding those responsible. Couldn’t have that happen again. Therefore, you now had those hopeful about HCQ and those who did not want HCQ to be so, and those fighting for the possible money on either side. Both studies from the University of Minnesota are used to close down any discussion of Hydroxychloroquine and Covid-19.
The “Hydroxychloroquine does not work” studies.
The first study out of the University of Minnesota is the Hydroxychloroquine in Nonhospitalized Adults With Early Covid-19 – A Randomized Trial. The conclusion was
Hydroxychloroquine did not substantially reduce symptom severity in outpatients with early, mild COVID-19.
But what is missed in science and health reporting is the limitations of the study which I believe are important. (My Underline)
Our original end point was an ordinal outcome of reduced hospitalization, intensive care unit stay, or death. Among the enrolled participants, the incidence of hospitalization was only 3% and incidence of death only 0.4%, making the planned analysis of the ordinal endpoint futile.
Our population was relatively young with 77% of participants being aged 50 years or less, with few comorbid conditions; thus, our trial findings are most generalizable to such populations. It is possible that hydroxychloroquine is more effective in populations at higher risk for complications, such as older persons in long-term care facilities (23). Performing randomized trials in long-term care facilities could test whether hydroxychloroquine can reduce hospitalizations
This trial may not inform whether an effect would be observed in populations at higher risk for severe COVID-19. Further randomized controlled clinical trials are needed in early COVID-19.
The second study out of the University of Minnesota is the A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. The conclusion was
After high-risk or moderate-risk exposure to Covid-19, hydroxychloroquine did not prevent illness compatible with Covid-19 or confirmed infection when used as postexposure prophylaxis within 4 days after exposure.
But what is again missed in science and health reporting is the limitations of the study which again miss the purpose of using the medication. ( Parenthesis and underline is mine)
We hypothesized that hydroxychloroquine could potentially be used as postexposure prophylaxis, to prevent symptomatic infection after exposure to Covid-19.
…the predictive power of this case definition (definition of what a Covid-19 positive case is by symptoms) is unknown, particularly in the younger populations that we studied; given the small number of PCR tests, it remains theoretically possible that hydroxychloroquine therapy limits proven infection. Reproduction of our results in other, ongoing trials would confirm our findings.
This randomized trial did not demonstrate a significant benefit of hydroxychloroquine as postexposure prophylaxis for Covid-19. Whether preexposure prophylaxis would be effective in high-risk populations is a separate question, with trials ongoing. In order to end the pandemic, a reduction in community transmission is needed.
About 70% of participants were again less than 50 years of age with minimal co-morbidities. Dr. Boulware and colleagues state that the use of hydroxychloroquine is to reduce symptomatic infection. I agree. We were originally told to “flatten the curve” to stop our healthcare facilities from being overwhelmed. But the vast majority of those pressuring our health systems were over the age of 50 with co-morbidities. In the end, they state “a reduction in community transmission is needed”.
By June we could see that this was harmful to a particularly vulnerable population (>50 years old with co-morbidities) and a large number of youth cases was not what was overwhelming our hospitals. Therefore, neither of these studies did what we needed them to do. Test the vulnerable populations to see if this would decrease the severity of the symptoms and therefore slow the hospitalization rate of the vulnerable population.
Remdesivir
Remdesivir is known for in vitro ( in the lab) antiviral results against SARS (2020) and MERS ( 2012) and Eboli. It did not work in vivo ( in humans). But, why not try this for the new Covid-19. This medication appears to stop the reproduction of the virus by halting the RNA production. See MedCram Feb 5, 2020. On February 4, 2020, a letter published in Nature advised looking at both Remdisivir and chloroquine.
Our findings reveal that remdesivir and chloroquine are highly effective in the control of 2019-nCoV infection in vitro. Since these compounds have been used in human patients with a safety track record and shown to be effective against various ailments, we suggest that they should be assessed in human patients suffering from the novel coronavirus disease.
In a June, 2020 article in the American Journal of Managed Care (AJMC),
Gilead Sciences Monday set the price for Remdesivir, its antiviral drug that can shorten hospitalization stays for individuals ill with coronavirus disease 2019 (COVID-19) from SARS-CoV-2 infection, at $520 a vial, or $3120 per patient for a typical treatment course, for those with private insurance.
As reported from AJMC, with taxpayer support, the National Institute of Allergy and Infectious Diseases funded a COVID-19 Remdesivir trial, and Patients for Affordable Drugs estimated that it will spend at least $30 million on further Remdesivir studies.
The Institute for Clinical and Economic Review (ICER) last week said remdesivir would be cost-effective at a price range of $4580 to $5080 if it saved lives. However, news that an inexpensive steroid, dexamethasone, which does not have a patent, improves survival means remdesivir should be priced between $2520 and $2800, ICER said.
100% of Medicare Part D and Medicare Advantage plans cover Hydroxychloroquine in general. Otherwise you can get this for about $6.00 for 60 tablets.
October, 2020
Skip ahead to October 15, 2020, The WHO SOLIDARITY trial was published showing no statistical benefit vs. placebo for treatment of either Remdesivir or hydroxychloroquine for improvement or death. Interestingly, there was a possible statistical improvement with patients on low flow oxygen meaning less severe or early in the infection or severity. This was not well reported but was reviewed with MedCram from October 16.
A side note is that for hydroxychloroquine and Remdesivir, the ages and other factors of severity of illness were not stratified. Therefore, the possible goal of reducing symptoms and severity before critical complications from Covid-19 infection arise or before hospitalization can not be known. Also, this SOLIDARITY study along with so many others did not combine chloroquine/ hydroxychloroquine with zinc. Unfortunately for Gilead Science, this was not done either, though I suspect no primary care physician is going to look at Remdesivir for early treatment with its price tag. The only way to recoup the investment is to have insurance pay for multiple days in the hospital. That is the benefit of a drug like hydroxychloroquine.
Dr. Anthony Fauci
From the Hill;
The overwhelming prevailing clinical trials that have looked at the efficacy of hydroxychloroquine have indicated that it is not effective in coronavirus disease.
January 2021
In the January edition of the Journal of Internal Medicine, Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection was published. This really is not any different from the initial “google doc” developed by James Todaro MD. Now there is a published, detailed possible algorithm for early treatment of patients before hospitalization.
We have proposed an algorithm based on age and comorbidities that allows for a large proportion to be monitored and treated at home during self-isolation with the aim of reducing the risks of hospitalization and death.
This is no different than hundreds of physicians were trying to investigate before they lost their jobs or kicked offline.
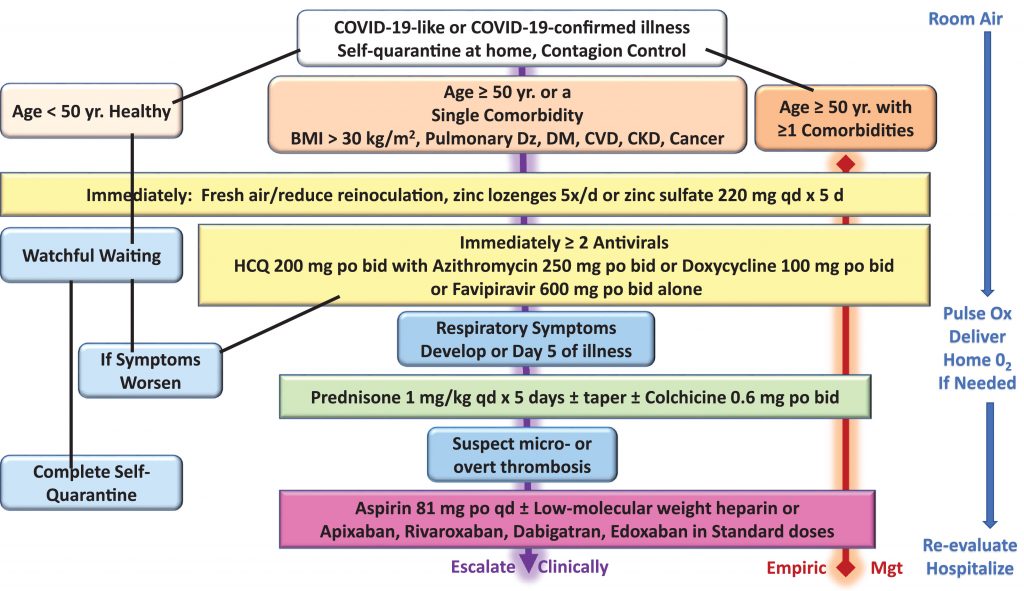
Medical Curiosity Lost
Hydroxychloroquine had been used for more than 90 years to treat patients of one type or another. The safety record from patients taking this drug has decades of data. The science of chloroquine/hydroxychloroquine against viruses and bacterium is present. The science of hydroxychloroquine alone and assisting Zinc in working against viruses are present. The cost is in the cheap.
In 2020, the above information was disregarded and so was any medical curiosity in a drug that most poor patients could get at a pharmacy with a prescription.
From WIRED article,
Some researchers still think the drug might have a small, as yet unproven effect if used early enough, or in a different amount. It’s possible, and it’s also possible no one will ever know. That would be normal. Part of knowing how to know stuff is knowing what the edges are. All science is settled, until it isn’t.